Viral Vector Manufacturing

VectorBuilder has extensive expertise in producing many different types of viruses. We can provide viruses of different scales and quality attributes to meet the full range of demands along the gene therapy drug development pipeline. We have established and validated platform technologies for large-scale GMP manufacturing of adeno-associated virus (AAV) and lentivirus. We also have experience producing other types of viral vectors such as adenovirus, MMLV, herpes simplex virus (HSV), and vesicular stomatitis virus (VSV). We can produce viruses in several grades that cover different downstream applications including drug discovery research, pre-clinical studies, clinical trials and commercialization.
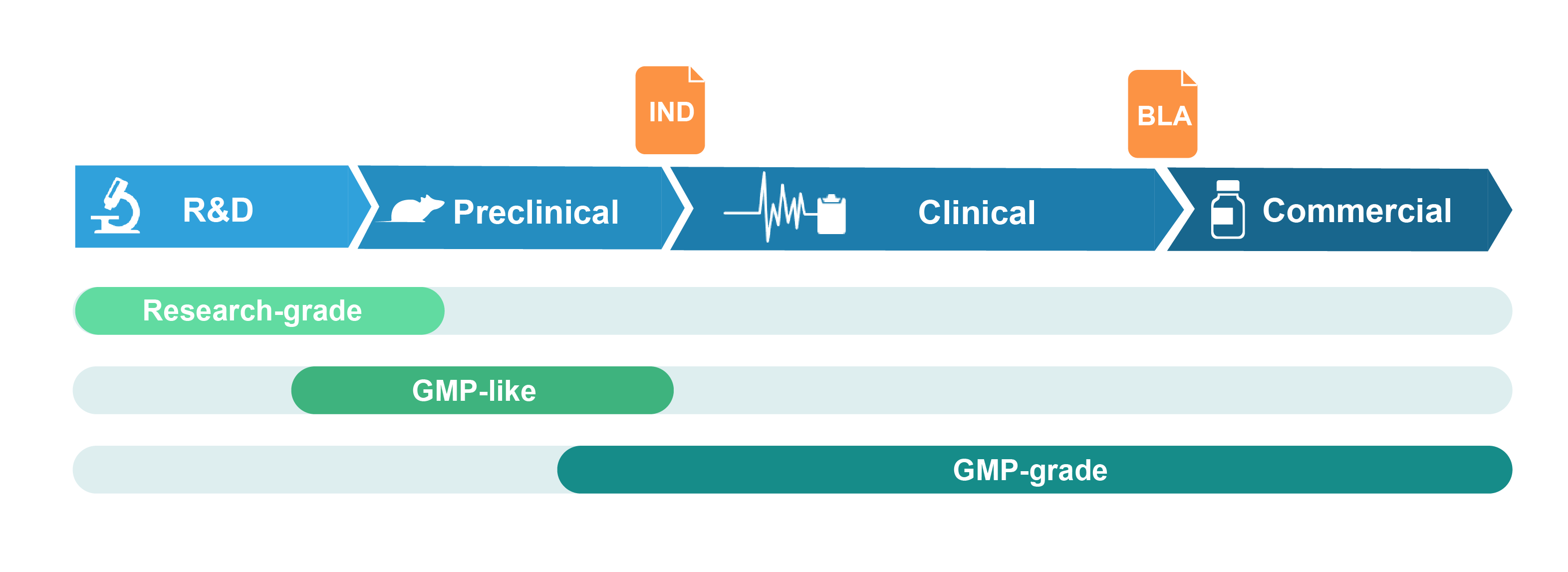
Grades of Viral Vectors Offered
-
Research-grade virus
Research-grade virus is suitable for basic research and drug discovery studies. It is produced in conventional cell culture facilities. Stringent QC assays are performed to ensure the quality of the virus meets the standards requested by customers.
Learn more about our research-grade virus -
GMP-like virus
GMP-like virus is suitable for pre-clinical studies including drug safety and metabolism testing in animals. The manufacturing of GMP-like virus follows key features of GMP guidelines, such that the production process and quality attributes are comparable to GMP with document control and traceability. GMP-like grade is therefore intended to be a small-scale mimic of the final GMP product, but with significantly lower cost and much faster timeline. Product release is accompanied by a certificate of analysis (COA). TSE/BSE statement can be provided upon request.
-
GMP-grade virus
GMP-grade virus is produced in our certified GMP cell culture suite following strict GMP guidelines. A comprehensive quality assurance system is embedded throughout the manufacturing process, including an array of in-process and release QC assays to ensure that the virus meets customer specifications and regulatory standards for quality and safety. At product release, a COA and a batch release report fully documenting the production process are provided.
Comparison of different grades of virus
| Research-grade | GMP-like | GMP-grade | |
|---|---|---|---|
| Applications | Basic research, drug discovery, and preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism | Preclinical studies, clinical studies, and commercialization |
| Production scales | 5x1010 to 1014 GC per batch | 5x1013 to 1017 GC per batch | 1014 to 1017 GC per batch |
| Turnaround time | 10-50 days | 4-5 months | 6-12 months |
| Production site | In parallel production in standard BSL-2 laboratory | Produced in segregated BSL-2 production suites | Produced in certified GMP suites (BSL-2) |
| Quality system | ISO9001 | ISO9001 while adopting key features of GMP manufacturing | ICH quality guidelines for GMP manufacturing |
| Document control and traceability | No | Yes | Full traceability |
| Vector characterization | RE digestion, ITR sequencing | Sequencing | Sequencing |
| Process development | No | Performed on a case-by-case basis depending on individual project needs | Yes |
| Cell banking | No | Available upon request | Yes |
| Antibiotic-free | No | Available upon request | Yes |
| Animal component-free | No | Available upon request for suspension culture | For suspension culture |
| Purification | Ultracentrifugation | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography |
| QC and release tests | Titer measurement, SDS-PAGE, endotoxin detection, sterility testing, mycoplasma detection, etc. | Performed on a case-by-case basis depending on individual project needs | Full panel QC assays, analytical development upon individual project needs |
| Aseptic fill/finish | No | Available upon request | Yes |
| Storage of retain sample | Available upon request | Available upon request | Yes |
| Document deliverable | COA | 1. COA 2. Manufacturing summary 3. TSE/BSE statement upon request |
1. COA 2. TSE/BSE statement 3. CTD documents 4. Others (BMR etc.) upon request |
| Research-grade | GMP-like | GMP-grade | |
|---|---|---|---|
| Applications | Basic research, drug discovery, preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism | Preclinical studies, clinical studies, and commercialization |
| Production scales | >2.5x107 TU | 109 to 1012 TU per batch | 5x109 to 1012 TU per batch |
| Turnaround time | 8-16 days | 4-5 months | 6-12 months |
| Production site | In parallel production in standard BSL-2 laboratory | Produced in segregated BSL-2 production suites | Produced in certified GMP suites (BSL-2) |
| Quality system | ISO9001 | ISO9001 while adopting key features of GMP manufacturing | ICH quality guidelines for GMP manufacturing |
| Document control and traceability | No | Yes | Full traceability |
| Vector characterization | No | Sequencing | Sequencing |
| Process development | No | Performed on a case-by-case basis depending on individual project needs | Yes |
| Cell banking | No | Available upon request | Yes |
| Antibiotic-free | No | Available upon request | Yes |
| Animal component-free | No | Available upon request for suspension culture | For suspension culture |
| Purification | Ultracentrifugation | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography |
| QC and release tests | Titer measurement, sterility testing, mycoplasma detection, etc. | Performed on a case-by-case basis depending on individual project needs | Full panel QC assays, analytical development upon individual project needs |
| Aseptic fill/finish | No | Available upon request | Yes |
| Storage of retain sample | Available upon request | Available upon request | Yes |
| Document deliverable | COA | 1. COA 2. Manufacturing summary 3. TSE/BSE statement upon request |
1. COA 2. TSE/BSE statement 3. CTD documents 4. Others (BMR etc.) upon request |
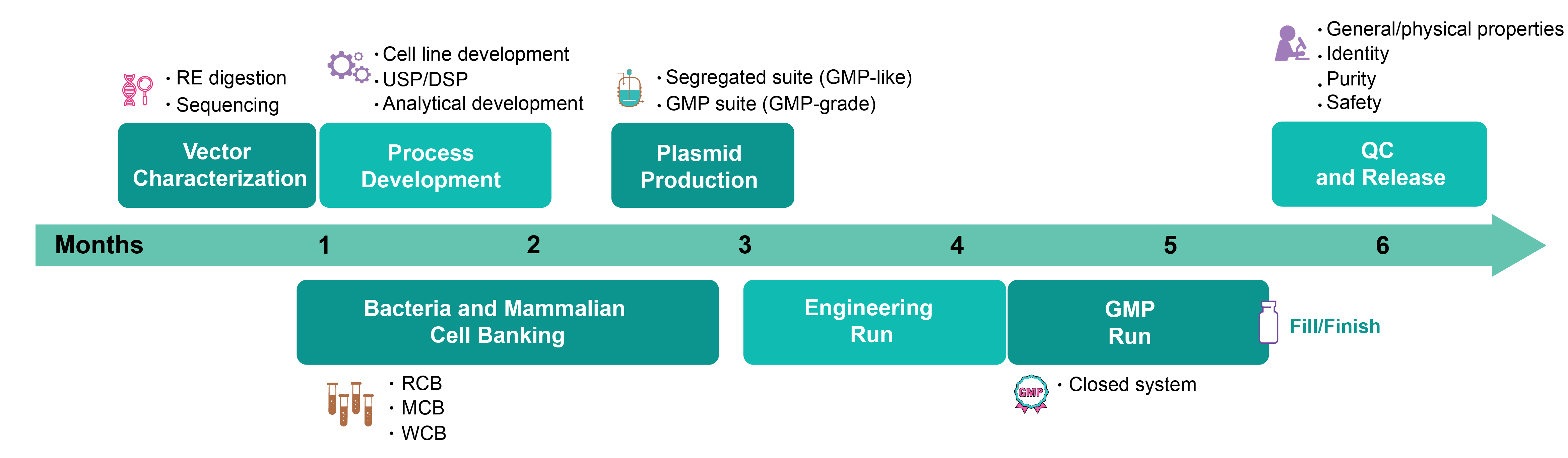
Workflow for GMP Production of Viral Vectors
Platform Technologies
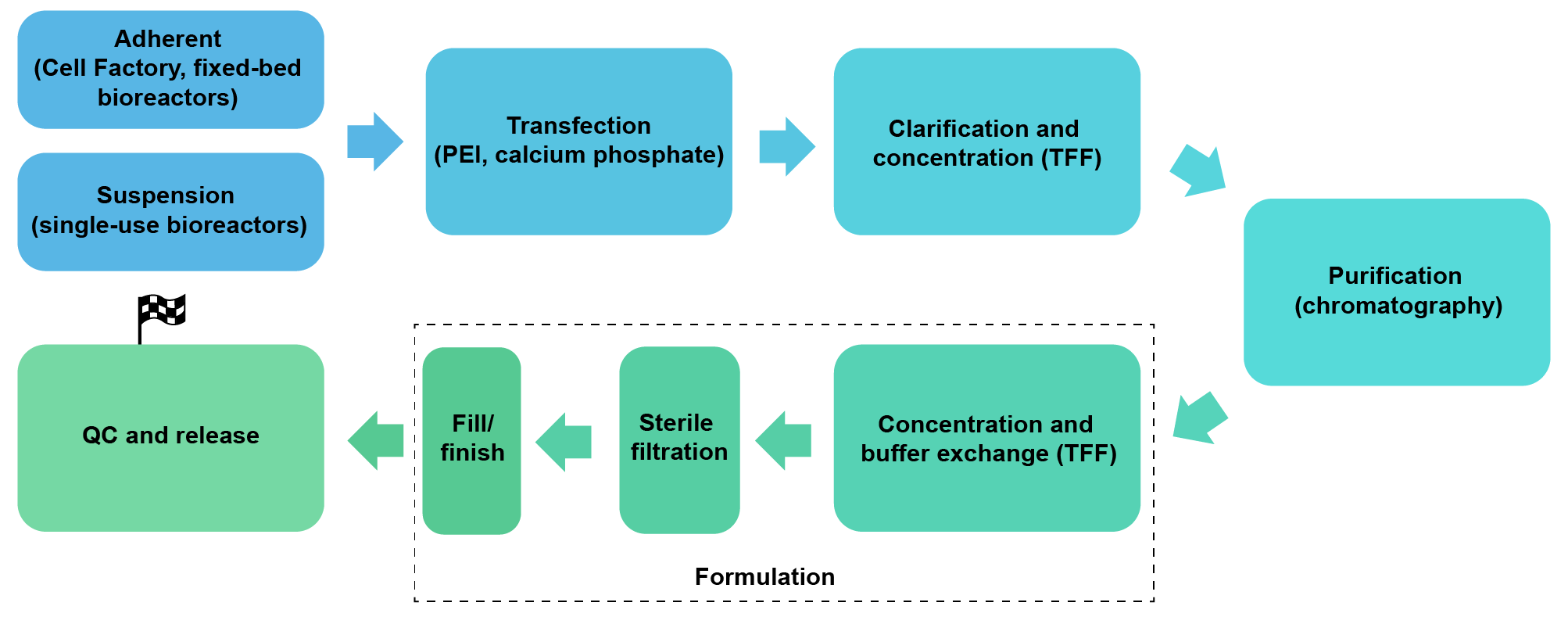
We package AAV in HEK293 cells under either adherent conditions (Cell Factory or fixed-bed bioreactors) or serum-free suspension conditions (up to 200 L single-use bioreactors). We also package AAV in suspension Sf9 insect cells. We can achieve a scale of up to 1017 GC AAV per batch.
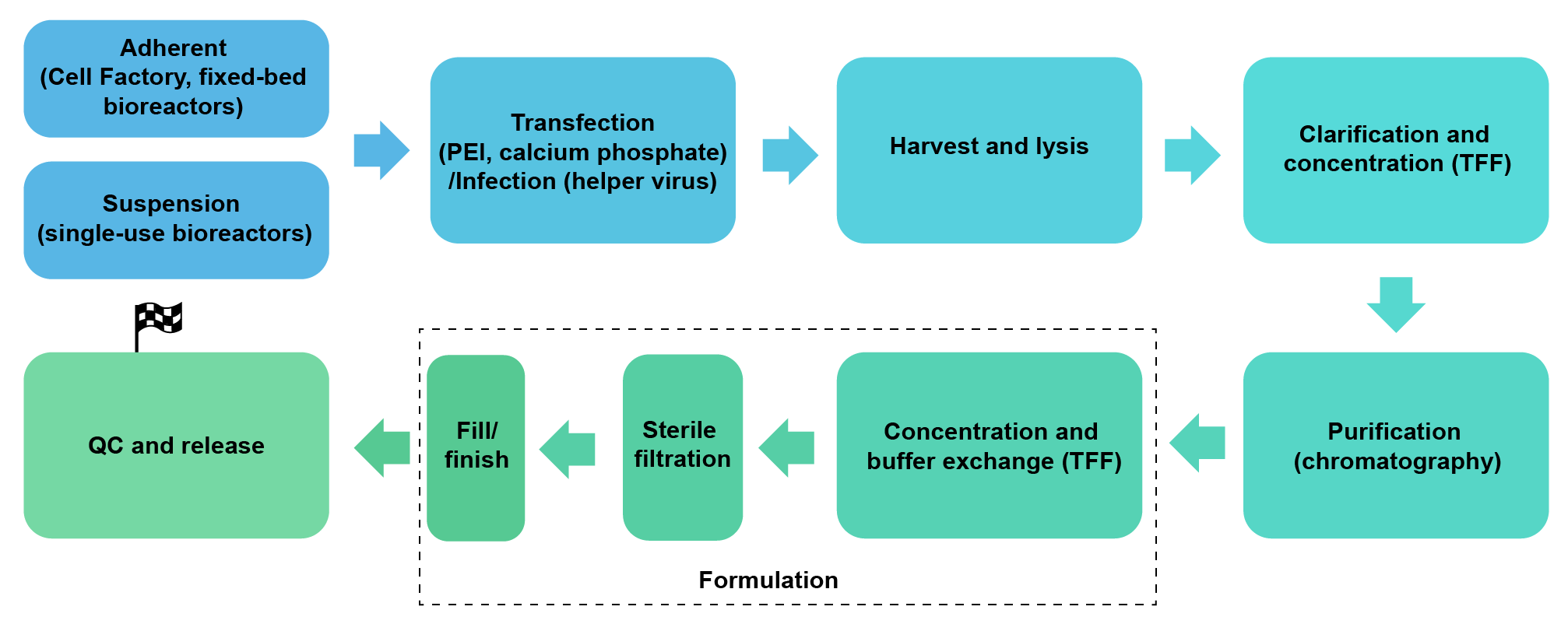
We package lentivirus (2nd and 3rd generation, pseudotyped with VSV-G or other viral surface proteins) in HEK293, under either adherent growth conditions (Cell Factory or fixed-bed bioreactors) or serum-free suspension conditions (up to 200 L single-use bioreactors). We can achieve a scale of up to 1012 TU per batch.